Distinguishing characteristics
of primary cells
Primary cells are isolated by mechanical and/or enzymatic dissociation from tissue biopsies of relevant donor organisms that are capable of propagation under certain permissible conditions ex vivo without further modification. This minimal manipulation is the principal defining characteristic of primary cells, as opposed to oft-employed continuous cell lines, which are modified to permit indefinite propagation in a procedure known as immortalization.1
In nature, differentiated somatic cells are subject to a process known as senescence whereby each chromosome replication event leads to progressive telomere shortening and, thus, an exhaustible replicative potential.2 Having not undergone immortalization, primary cells recapitulate this limitation, but in turn exhibit characteristics and behaviors more closely aligned with that of the equivalent cell in its native physiological context. Several studies have identified changes in gene expression, immune privilege, apoptosis signaling responsiveness, and adenovirus transduction efficiency, all as a result of immortalization.3 4 5 6 7 Immortalization fundamentally alters the overall behavior of a cell to prioritize propagation, an intensely resource-demanding process, to the likely detriment of processes not in direct service of this goal.
Like the constituent cells of a given tissue, primary cell populations are interrelated but clonally impure. Unlike continuous/immortalized cell lines, which are derived from a single common ancestral cell and thus give rise to a homogeneous population of clones, a primary cell culture is established from a population of cells isolated from its native host tissue which may exhibit differences in gene expression and degree of differentiation. Moreover, depending on the effectiveness of isolation techniques as well as the makeup of the tissue sample, the initial primary cell culture may contain cells or cell populations of distinct subtypes also present in the tissue sample. Culture conditions may be chosen to favor propagation of the population of interest, but some heterogeneity may still exist. For this reason, experiments are often conducted using clonally pure immortalized cells in order to enhance consistency and reproducibility. This choice, however, is a double-edged sword; whereas robust reproducibility is certainly desirable, it is arguably surpassed in importance by biological relevance, a metric by which primary cells are superior.
Still, continuous/immortalized cell lines remain valuable, cost effective tools, particularly as applied in the rapid, iterative experimentation that is often necessary at the outset of a novel investigative avenue. As with any other experimental approach, however, orthogonal confirmation of results is paramount, a need that is optimally satisfied by replication of results in a more relevant primary cell culture.
Advantages and disadvantages of primary and continuous cell culture
The superior physiological relevance relative to their immortalized counterparts is the principal and most well-known advantage of primary cells in biomedical research. While immortalized cell lines may be, and routinely are, applied to delineate cellular mechanisms and disease phenotypes, care should be taken to thoroughly characterize any cell line employed for this purpose to provide a measure of confidence in the relevance of the model system in the context of a particular investigation. Misidentification, cross contamination, and genetic drift of continuous/immortalized cell lines are unfortunate side effects of their ease of cultivation and capacity for indefinite propagation. Researchers are often unaware of these risks, and thus fail to properly control these potential impacts to a study by careful cell line selection, authentication, and characterization prior to application.8 9 10 11 12 The scientific literature abounds with studies using misidentified or contaminated cell lines leading to invalid conclusions.13 14 15 16 17 18 Moreover, the indefinite propagative potential of immortalized cell lines, one of their greatest perceived strengths, has led to the widely accepted presumption that passage number is irrelevant. However, while several studies have refuted this notion,19 20 21 22 23 studies utilizing excessively subcultured cells are still frequently reported.24
Primary cells, with their inherently limited lifespans, do not invite such abuses. Moreover, the naturally limited duration of cultivation and ability to continuously replenish cell stocks from source tissue decrease the risks of misidentification and cross contamination with other cell types and opportunistic organisms such as mycoplasma. This advantage, of course, is dependent upon approriate laboratory conditions and precautions. Genetic drift is also curtailed by the senescence of primary cells; indeed, it is the propensity of indefinitely dividing cells to become harmful to the host organism that has led to the evolution of cellular senescence.25 Clonal heterogeneity in populations of somatic cells is a well-known phenomenon.26 27 28 Whereas immortalized cell lines are principally identical, notwithstanding genetic drift over the course of cultivation, primary cell populations can sometimes recapitulate the phenotypic variation encountered in their originating host tissues, although controlled and well-defined techniques and culture conditions can favor certain types of cells over others, leading to enrichment of a particular subpopulation, such as myoblasts.
Soon after culture establishment, primary cell cultures tend to become dominated by rapidly expanding fibroblast-like cells to the detriment of slower growing primary cell type.29 Therefore, depending on the cell type of interest, it is recommended to utilize unpassaged or early passage primary cells unless it can be demonstrated that the cell type of interest prevails at the passage number chosen. Carefully characterized media can also help select for the cell type of interest.
Whereas immortalized cell lines can often be maintained successfully in conventional media supplemented with bovine serum, primary cells tend to require more optimized growth conditions and often depend upon tissue specific cytokines and growth factors for productive cultivation.30 For well characterized primary cell types, these growth conditions are often described in the literature and/or by the commercial or institutional provider.
Cells for muscle related research
In muscle cell research, many investigators have used the immortalized C2C12 mouse cell line first described in 1977 for elucidating pathways of muscle cell biochemistry and certain aspects of growth regulation.31 While much has been accomplished with C2C12 cells, understanding human muscle differentiation and muscle related diseases highlight the need for appropriate human muscle cells. For human cell research, current options include immortalized cell lines developed by some research groups,32 33 iPSC derived human myoblasts from commercial suppliers, and primary skeletal muscle derived cells (skMDCTM) which are available from several suppliers, including Cook MyoSite.
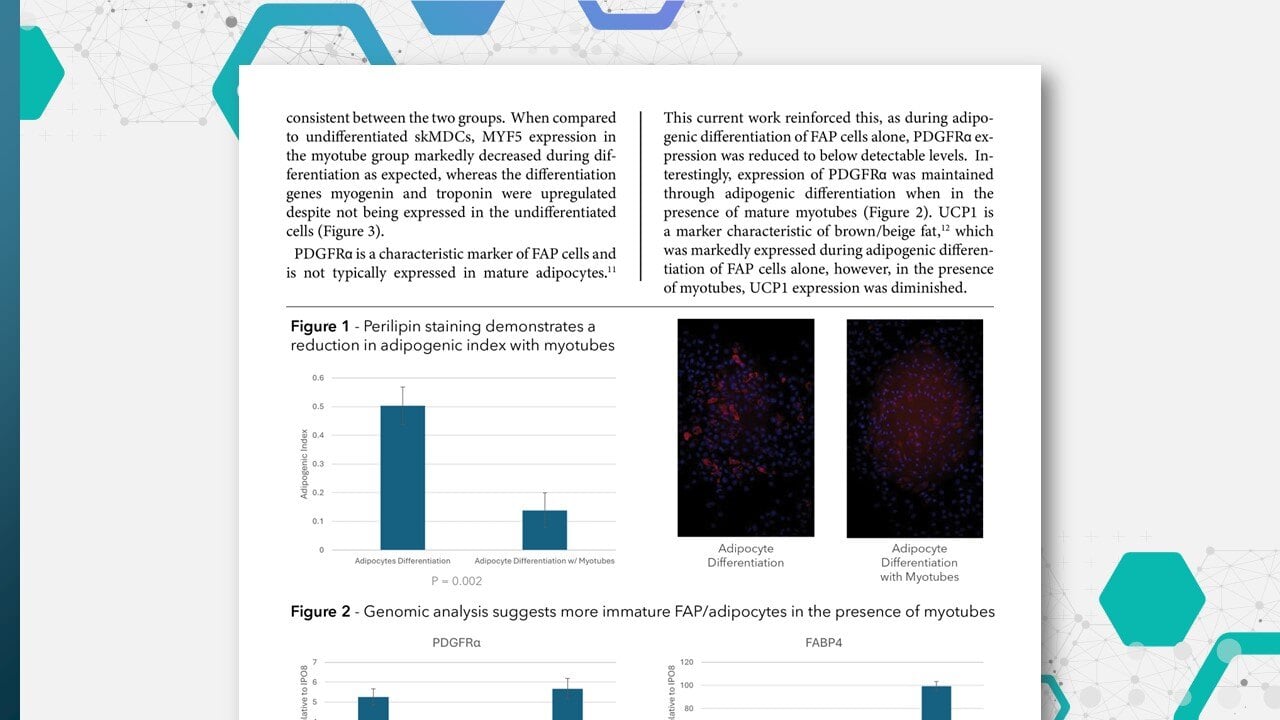
Cook MyoSite provides low passage muscle cells from a wide variety of individual donors. MyoSite skMDCs typically demonstrate doubling times of 24 hours and can be cultured out to 12 or more passages.
Healthy MyoSite skMDCs are all well characterized for sterility, viability and desmin positivity (Fig. 1), and are also capable of robust differentiation into myotubes under appropriate conditions (Fig. 2) thus providing a highly relevant and reproducible model to study muscle differentiation and disease. It has also been noted that differentiated MyoSite skMDCs can be efficiently transduced with adenovirus.34
Common misconceptions regarding primary muscle cells are that primary cells are expensive and inaccessible. The notion that primary cells are much more expensive and difficult to acquire may cause researchers to limit their studies to experimentation in a continuous/immortalized cell line. In fact, the cost incurred by primary cell research models relative to continuous/immortalized cell lines is worth the expenditure due to the inherent advantages of using a more native/relevant biological model.
Assuming equivalent seeding and passage densities as well as a sensible maximum passage number for immortalized cells, primary cells such as MyoSite skMDCs are only 2-3 times more expensive than mouse C2C12 cells, and less than the cost of iPSC derived cells. MyoSite skMDCs offer the benefits of a more relevant biological model to tackle complex biological investigations.
As for accessibility of primary cells, the catalogue of skMDCs and compatible growth, differentiation, and cryopreservation media products offered by Cook MyoSite allows researchers to apply these high-quality cell samples without the hurdles of tissue biopsy sourcing, appropriate growth condition determination, and primary culture establishment.
Discussion
Primary cells have numerous advantages over continuous/immortalized cell culture models, but are also associated with a number of challenges that must be considered and addressed in order to increase the likelihood of success. Choosing an appropriate cell type, determining optimal culture conditions, characterizing relevant aspects of cell behavior in the model system of interest, and rigorous experimental planning to ensure sufficient supply of similarly passaged cells are essential. However, if applied correctly, primary cells can enhance any study in immortalized cell lines by corroborating indications and observations.
Cook MyoSite can provide large quantities of primary human muscle cells derived from donor tissue using well established and consistent methodologies for the cultivation of myoblasts for use in cell-based assays and other applications.34 MyoSite has a portfolio of compatible media products and a large library of well-characterized, consistently performing primary skMDCs. In addition to normal/healthy donors, Cook Myosite also offers primary skMDCs from donors afflicted by a variety of neuromuscular and certain other diseases (i.e. Muscular Dystrophy, Chronic Fatigue Syndrome, Myasthenia Gravis, ALS, Guillain-Barre Syndrome, Marfan Syndrome) for studies of cellular disease mechanisms and drug development.
In considering overall resources needed for biochemical and cell based assays using human muscle cells, Cook MyoSite skMDCs and compatible media products can be cost effective tools. skMDCs can provide increased overall efficiency by enabling investigators to sidestep initial tissue sourcing, cell isolation, and culture condition optimization, which are time and labor intensive undertakings. Studies utilizing primary cells throughout can provide valuable and important answers that immortalized cells may not be able to, thereby ensuring confidence in the outcomes from beginning to end.
Get in touch
Complete this form and one of our product experts will connect with you shortly.
Investigators and study participants currently involved in one of the Cook MyoSite, Inc. clinical trials
should not
use this contact form.
If you are a study participant currently involved in one of the Cook MyoSite, Inc. clinical trials, please instead contact your treating physician.
If you are a study participant currently involved in one of the Cook MyoSite, Inc. clinical trials, please instead contact your treating physician.